As many of you may have heard in recent times, delivery of vaccines can be extremely challenging. It is necessary to store and transport all vaccines within a specific range of temperature. Many different organisations, technologies and people work together to make this possible and this is where cold chain monitoring comes in.
Establishment of a strong cold chain is essential for the storage, transportation, and overall management of any temperature-controlled product. A recent study has revealed that over 30% of global pharmaceutical deliveries are spoiled or damaged before reaching their destination. This can only be improved by ensuring the cold chain management process is more effective.
Cold Chain and its Importance
A cold chain may be defined as a temperature-controlled supply chain for the effective handling of temperature-sensitive goods or products. It comprises of an unbroken series of refrigerated processes for perishable items, covering production, storage, transport, and distribution. The objective of a cold chain is to ensure that the products are able to retain their desired qualities when they reach the end user.
The process of monitoring a cold chain is known as cold chain monitoring. This is done using different technology tools such as temperature systems and data loggers, etc. Please note that the terminologies, cold chain and cold chain monitoring are often used interchangeably.
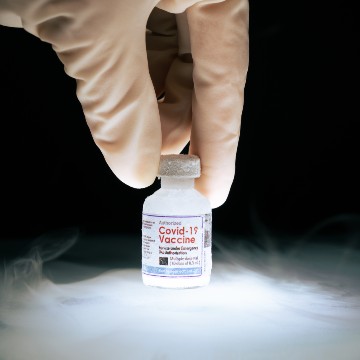
When it comes to cold chains for vaccines, all of these need to be stored within a specific range of temperature. This chain starts from the time of manufacturing and ends when the vaccine is received by the recipient. The cold chain temperature requirement for some vaccines can be as low as -80°C.
To safeguard the quality and reliability of vaccines, they must remain within a specific temperature range, right from the time of production through to storage and transportation. Therefore, the vaccine cold chain is all about the establishment of a temperature-controlled supply chain for the storage, handling, transportation, and distribution of the vaccines.
Vaccine Cold Chain Management
The most important factors for cold chain management of vaccines in summary are:
- Storage temperature: As already mentioned, vaccines must be stored as per the recommendation of the manufacturers. The quality of vaccines may be adversely affected by temperature fluctuations and use of inappropriate storage equipment. Some vaccines may even require a frozen environment for storage.
- Plan for storage and handling: A plan for vaccine storage and handling should be created, covering orders, accepting deliveries, inventory management, vaccine storage and handling and management of compromised vaccines.
- Training and education: Sufficient training must be provided to all personnel handling the vaccines so that they are aware of all policies, procedures, and techniques required.
Temperature Monitoring in Cold Chain:
In order to prevent product deterioration resulting from temperature fluctuations, temperature indicators and temperature recorders are used extensively. Temperature indicators are inexpensive devices capable of providing a visual indication when the temperature has gone above or below the acceptable temperature range. Temperature recorders are more sophisticated and can be used for continuous temperature tracking. They can be used to simply retrieve temperature data or transmit the stored temperature data using different connectivity technologies.
At ShockWatch, we offer an advanced range of vaccine temperature monitoring products that have been used with great results in the management of the cold chain for vaccines.
Please contact us today to find out more.